Who Should Get the RSV Vaccine?
Babies and older adults are eligible for this newly available protection
TL;DR: RSV vaccines can dramatically reduce the disease burden for babies and older adults.
While RSV (respiratory syncytial virus) doesn’t get quite as much media attention as COVID-19 or the flu, it’s still a respiratory virus to watch out for. While it feels like a “common cold” for many, RSV can quickly turn dangerous for babies and older adults. RSV is highly contagious, spreading easily both through the air and from virus left on hands and surfaces (making it especially easy for kids to spread).

RSV infects the lower lungs and can lead to severe complications like pneumonia and bronchiolitis (inflammation of the airways in the lungs). RSV is the leading cause of infant hospitalizations in the US, and normally causes more than 2 million doctor’s visits in kids under age 5 annually. RSV also causes 110,000-180,000 hospitalizations a year for adults aged 50 and over. RSV activity typically picks up in the fall and peaks in the winter– so pretty much now.
That’s the bad news. The good news is that we have new tools to dramatically reduce the risk of severe illness from RSV. 2023-24 was the first season RSV vaccines were available, so not everyone has these on their regular fall vaccine radar. We now know that these vaccines have good safety records and are incredibly effective — so now is a great time to get protected before this RSV season picks up.
Who can get RSV protection?
In the US:
Adults aged 50 and over: The US CDC and the American Academy of Family Practitioners recommend the RSV vaccine for all adults aged 75 and over, and adults aged 50-74 who are at increased risk of severe RSV (including diabetes, cardiovascular disease, chronic lung disease, and immunosuppression). If you got an RSV vaccine in the last two years you do not need to get one now (they are expected to protect for at least two years and no additional doses are currently recommended, so stay tuned).
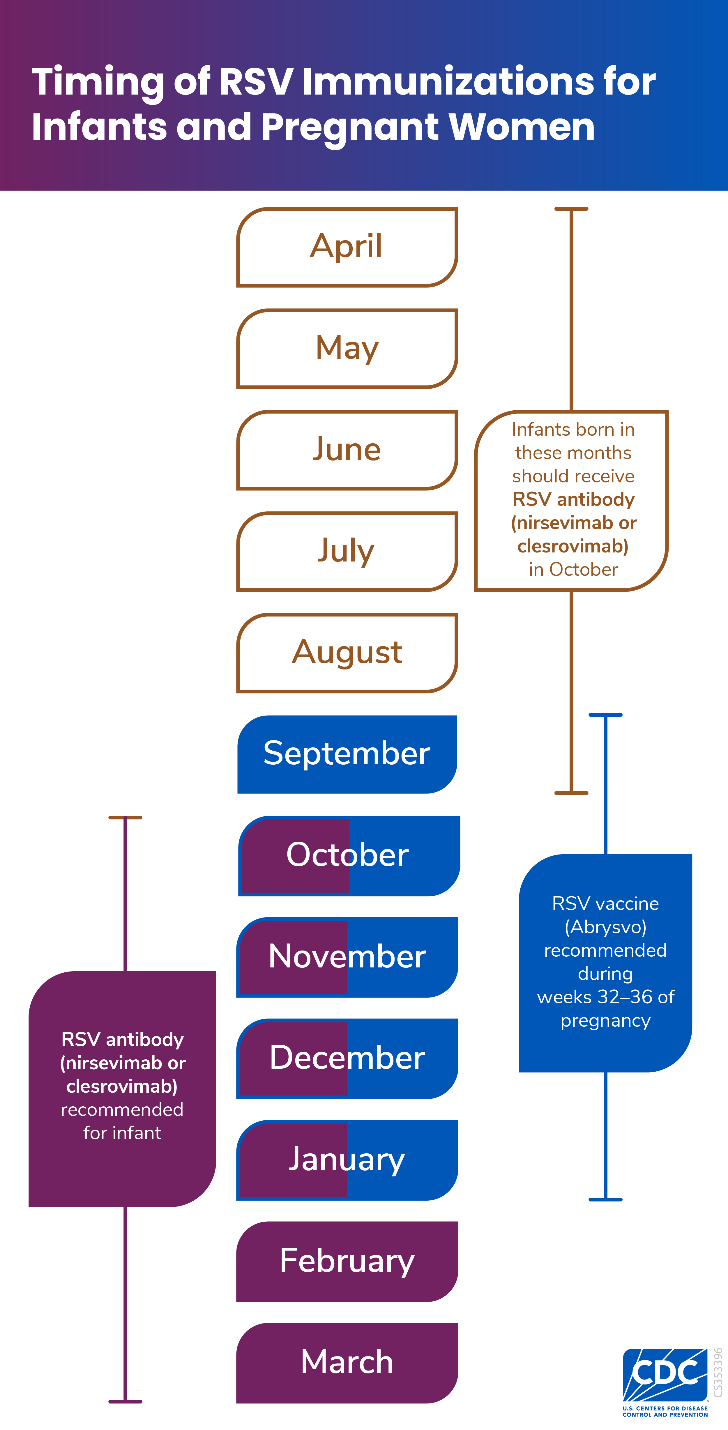
Infants: Babies have TWO different options for getting protected. The CDC and the American Academy of Pediatrics recommend:
Maternal vaccination (brand name Abryvso) between 32-36 weeks gestation—maternal antibodies to RSV are then passed to the baby and protect them for up to 6 months during their first (and most dangerous) RSV season.
RSV antibody for the baby– An RSV antibody called nirsevimab (brand name Beyfortus in the US) is recommended for all babies <8 months whose mothers did not receive the RSV vaccine during pregnancy. Beyfortus is a monoclonal antibody rather than a vaccine, meaning the baby gets passively protected by providing them the antibodies against the virus. It has the same effect as a vaccine in providing protection against infection. The dose should be given to babies shortly before the RSV season, or within 1 week after birth if born between October and March (see timing chart, below).
Either option will give your baby good RSV protection– so don’t overthink, just get that protection!
In the UK, you can get a free RSV vaccination from the NHS if:
you’re 28 weeks pregnant or more
you’re aged 75 to 79
Babies in the UK born prematurely or otherwise at increased risk will be offered the RSV antibody nirsevimab for the first time during the 2025-26 season.
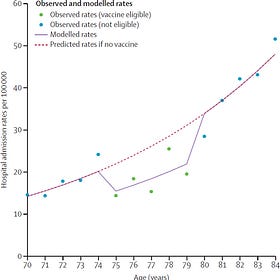
Analysis of the 2024 RSV vaccine roll-out in England found a significant reduction in hospitalizations among older adults, which I wrote about a few months ago:
Some Good News: New RSV Vaccine Reduced Hospitalizations in Older Adults
We’re starting to see the real-world impact of the new RSV vaccine, which protects against respiratory syncytial virus (RSV).
A Public Health Win
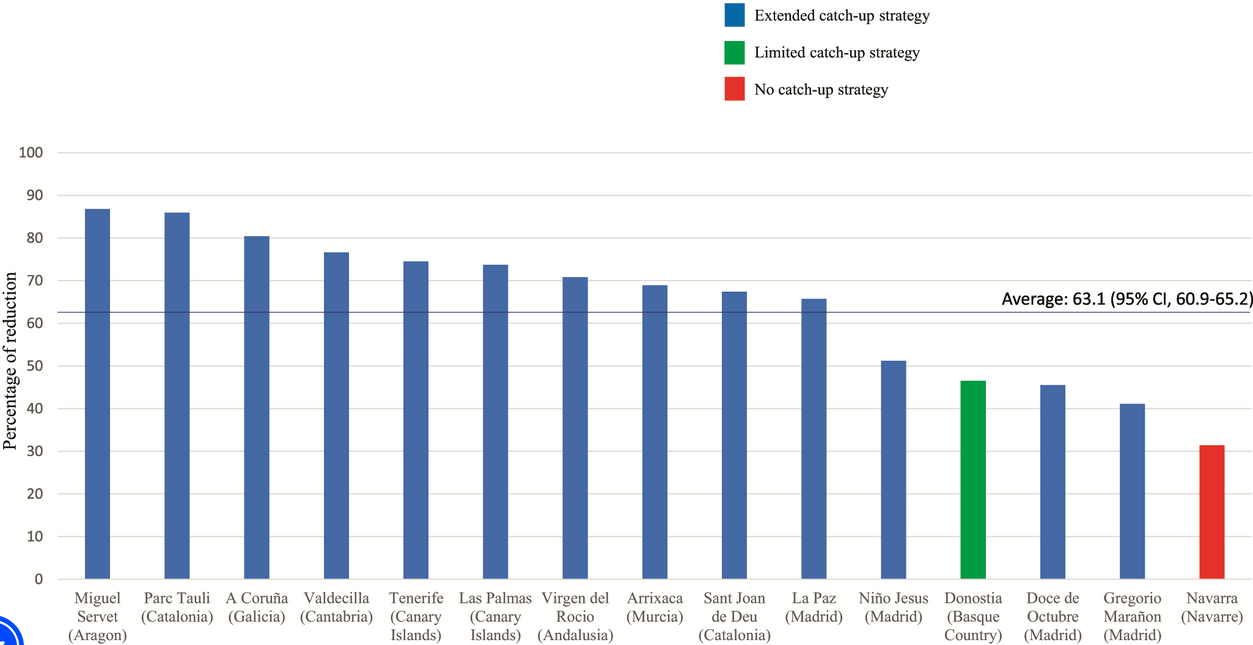
We got some stunning real world evidence on the effectiveness of this new protection for infants in the 2024 RSV season in countries that made it a big part of their routine newborn immunizations. Spain introduced universal RSV antibody shots last year for infants born during RSV season, as well as “catch-up” shots for all infants under 6 months. Some regions didn’t fully implement this “catch-up” recommendation. This study compared the RSV hospitalizations in each region compared to prior years without the RSV campaign. On average, the new policy was associated with more than a 60% reduction in infant hospitalizations and ICU admissions, and this effect was higher in regions with better “catch-up” policies for young infants not given the shot as newborns.
Percent reduction in RSV hospitalizations compared to previous seasons. Source: https://doi.org/10.1542/peds.2024-066584) Keeping babies out of the hospital for the win.
With all the bad news out there, it’s important to share good news when it happens. That includes giving a massive shout-out to all of the scientists who have spent careers dedicated to this– RSV vaccines have been fifty years in the making.
BOTTOM LINE
Science has done its part to deliver safe and effective RSV vaccines. Now it’s time to convert that hard work into real results. Go get your RSV protection if eligible, and encourage it for all the older adults and babies in your life.
Stay well,
Jenn