Some Good News: New RSV Vaccine Reduced Hospitalizations in Older Adults
Vaccines, getting the job done.
We’re starting to see the real-world impact of the new RSV vaccine, which protects against respiratory syncytial virus (RSV). RSV feels like a “common cold” for many but can quickly turn dangerous for babies and older adults. RSV infects the lower lungs and can lead to severe complications like pneumonia and bronchiolitis (inflammation of the airways in the lungs).
A new brief report in the Lancet looked at the roll-out of the RSV vaccine among older adults in England in the fall of 2024, finding a significant drop in hospitalizations for those eligible for the new vaccine.
You might remember the quirks of new vaccine roll-out in the UK which generated a cool natural experiment for this shingles vaccine and dementia study in Wales.
Given the universal health coverage in the UK, new vaccine recommendations are evaluated not just for effectiveness against disease but for cost-effectiveness for the government. The new adult RSV vaccine was deemed cost-effective for those aged 75 and over.
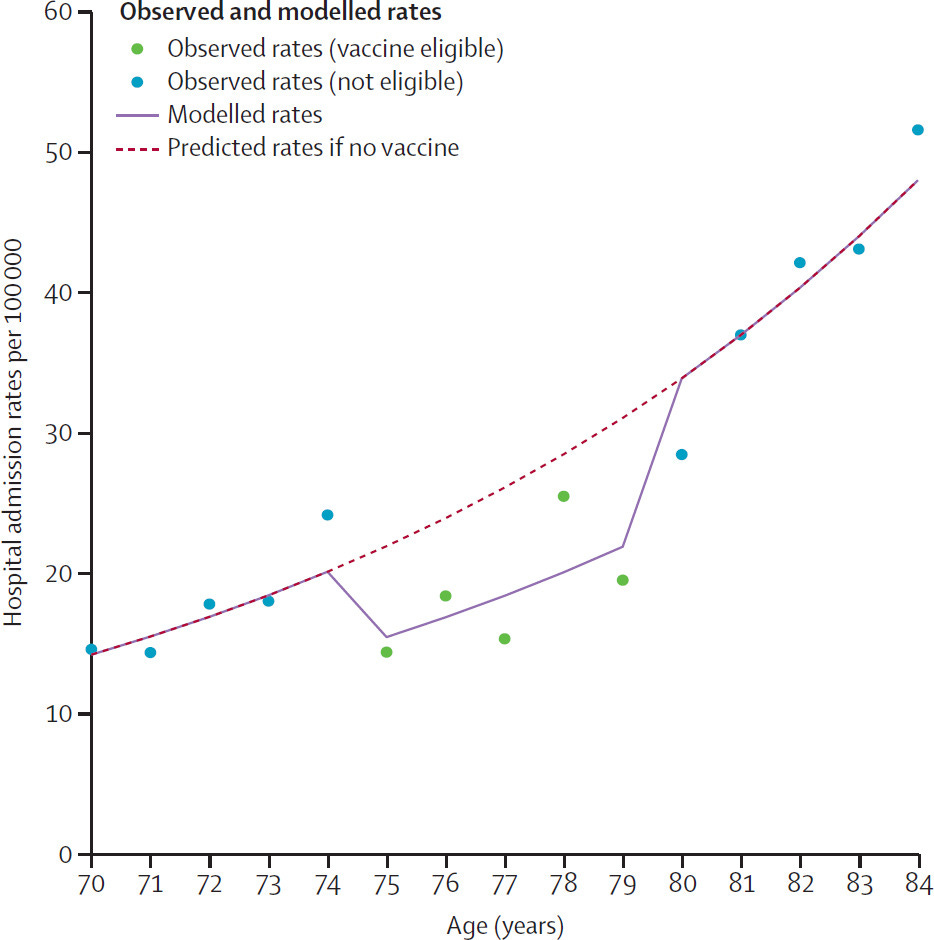
So, in the fall of 2024 adults aged 75-79 in England were eligible for the RSV vaccine for the first time. While I’m not clear on why those already over age 80 were out of luck, this quirk creates another “natural” experiment to examine vaccine effectiveness in the real world. In this case, the authors look at whether there was a break in the trend of RSV-associated hospitalizations around the age group eligible for the vaccine.
By the end of the study period in early January 2025, around 47% of people aged 75-79 had received the RSV vaccine. The researchers found a 30% reduction in RSV-associated hospitalizations in the eligible age group compared to what would have been expected based on the rates of people above and below the age cut-off:
Observed, modelled, and predicted RSV-associated hospital admission rates by age. Source: Mensah, et al, 2025. Early impact of RSV vaccination in older adults in England. The Lancet, Volume 405, Issue 10485, 1139 - 1140
Since less than half of eligible people actually got the vaccine, this 30% drop in hospitalizations would correspond to close to the 72% efficacy against severe disease seen in RSV clinical trials if everyone in that age group received the vaccine.
Overall, this is reassuring news that the protection seen in the clinical trials is translating into population-level effects.
This analysis was a much quicker and dirtier version of a regression discontinuity design (RDD) compared to the very thorough analysis in the recent shingles vaccine and dementia study. But given the specificity of the outcome (RSV-associated hospitalizations) and the narrow age range eligible, it’s a reasonable and convincing way to evaluate the impact of the vaccine roll-out. It’s hard to think of anything else besides the vaccine that could have made the RSV hospitalization rates differ only for 75-79 year-olds.
This data adds to the good news we got from last year’s RSV season. In 2023-24, Spain introduced universal RSV antibody shots for infants born during RSV season and “catch-up” shots for all infants under 6 months. Some regions didn’t fully implement this “catch-up” recommendation, providing another natural experiment by comparing before and after rates across regions. The new policy was associated with more than a 60% reduction in infant hospitalizations and ICU admissions on average, with bigger reductions in regions with better “catch-up” policies for young infants.
Keeping babies out of the hospital for the win: Percent reduction in RSV hospitalizations compared to previous seasons. Source: https://doi.org/10.1542/peds.2024-066584)
With all the bad news out there, I going to make an effort to share GOOD news when it happens. RSV vaccines were fifty years in the making, so a huge thanks to all of the scientists (and scientific funding) that helped make this happen.
We’re past RSV season for now, but I’ll remind you about these promising data next fall when it’s time to shore up your respiratory virus protection. The US Advisory Committee on Immunization Practices (ACIP) just voted to expand their RSV vaccine recommendation to adults aged 50-59 who are at high risk of severe RSV illness, but whether this will receive final approval from CDC is still TBD. Hopefully this good news will motivate the UK to offer the vaccine to those over age 80 as well.
Stay tuned, and stay well!
Jenn
In case you missed it:
Can the Shingles Vaccine Prevent Dementia?
You may have seen headlines about a fascinating new study in Nature suggesting that the shingles vaccine may lower the risk of dementia.